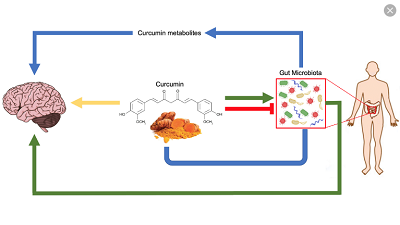
I decided to give the caption to this piece because of feedbacks from readers. The brain and the gut have a lively ongoing dialogue through the gut-brain axis. This was the introduction of paper by Madison et al that caught my attention recently. The title was “Stress, depression, diet and the gut microbiota: human-bacteria interactions at the core of psychoneuroimmunology and nutrition” published Current Opinion in Behavioural Sciences, 2019, 28:105-110. The ensuing is what I gathered from the piece. The paper noted that most people testified that negative emotions and stress affected gut motility (movement). Studies had shown that the gut-brain axis was relevant in longer-lasting conditions.
Digestive disorders such as irritable bowel syndrome usually coincided with mood disorders and showed an imbalance (dysbiosis) in the gut microbiota. There was therefore scientific interest in modulating the gut microbiota as a remedy for these disorders.
Independently and mutually, diet, stress and mood substantially influenced the survival of gut microbes. It was increasingly clear that environmental factors and health behaviours explained more microbiota variability than host genetics. Many modern practices such as antibiotic use, Western diet, high-stress lifestyles promoted gut bacteria imbalances, and low diversity (smaller count and uneven distribution of bacterial species). A balanced gut microbiota was important for good health and wellbeing. Imbalanced gut microbiota altered food cravings, metabolism, stress reactivity, mood and immune system dysfunction among others. In times of stress the autonomic and circulatory systems conveyed distress signals to the gut. A new bone marrow-mediated pathway highlighted the role immune cells played as messengers that conveyed psychological stress to the gut. The heightened inflammation that frequently accompanied stress and depression triggered blooms of pathogenic bacteria that caused an imbalanced gut microbiota and a leaky gut. It was important to note that chronic and acute stressors could shift the gut bacteria in multiple regions and habitats- both the inside (lumen) and border (mucosal lining) of the gut. Catecholamines, for example, elevated ceratin bacteria levels 10,000 fold and intensified their infectiousness in 14 hours. The resulting pathogenic species crowded out the beneficial species. A related study showed that as university students’ stress increased throughout the semester, certain health promoting bacteria decreased.
Stress and mood disorders reinforced each other. Bacteria which were proinflammatory species tended to dominate at the expense of health-promoting species in depressed individuals. Stress and depression increased gut barrier permeability resulting in a leaky gut that allowed bacteria to seep into circulation and produce an inflammatory response.
Stress and depression promoted unhealthy food choices and poor metabolic responses. Diet functioned as a major pathway from stress to gut dysbiosis. Even mild stressors could encourage unhealthy eating. Stress deactivated executive function in response to food cues and elicited a bias toward comfort foods. Stress and depression altered metabolic responses to food. Women with a history of depression had higher postprandial (after meal) cortisol and fat oxidation compared to women without a depression history. Diet was one of the most powerful predictors of gut bacteria composition- above and beyond one’s genotype. Diet determined which bacteria would thrive in the gut and the gut bacteria in turn aided digestion. It must be stressed that although long-term diets formed the gut community’s structure, dietary modification could produce detectable shifts in bacteria species of the gut microbiota within 24 hours.
Macronutrient profiles predicted unique gut microbiota populations- plant protein, unsaturated fats, fiber content all supported pro-health gut microbiota. This was in contrast to the high consumption of animal protein, saturated fats and refined artificial sugars.. Western diet with high saturated fat, processed foods, refined sugar fostered a distinct gut microbiota signature with low gut microbiota signature and greater gut leakiness- a precursor to metabolic syndrome and onset of chronic diseases. Low fiber consumption dysregulated immune function because short chain fatty acids (SCFAs) resulting from bacterial fermentation of complex carbohydrates were important for health immune function.About 60-70 million Americans suffer from digestive disorders with a cost outlay of about $100 billion annually. Furthermore, some digestive medications affect the gut microbiota. Proton pump inhibitors (e.g. Omeprazole) affected 20% of bacterial species causing imbalanced gut microbiota and in turn predisposing one to gastrointestinal infection. A balanced gut microbiota ensured that one species did not override the human host.
Imbalance gut microbiota prompted dysregulated eating behaviour in line with the dominant bacterial species needs. The gut bacteria influenced food choices through the production of molecules that mimicked or interfered with human appetite. The gut bacteria through the release of neurotransmitters (serotonin, acetylcholine, norepinephrine) indirectly influenced eating behaviours through mood changes. Targeted delivery of a bacteria produced SCFA, propionate, to the colon over 24 weeks reduced meal-size, weight gain, belly fat and increased post-prandial satiety signals in overweight adults. Healthy diet reduced the risk of depression. The gut microbiota influenced stress reactivity and mood.
Antibiotics have been found to cause imbalance in the gut microbiota. A study in the U.K. showed that a course of antibiotic, which destabilized the gut microbiota, increased risk for anxiety and depression by about 20%. Multiple courses of the antibiotic increased the risk of anxiety and depression by almost 50%. Antibiotic use programmed a new ‘set point’ of gut bacteria that was relatively stable even though it could facilitate poorer mental and physical health. The need for rational use of antibiotic use is critical in protecting the gut microbiota. Dear reader, you will appreciate the daily messages to increase your consumption of cocoa. Polyphenols modulate the gut microbiota. Cocoa is the richest food source of polyphenols on weight basis.
WISHING YOU ALL A MERRY CHRISTMAS AND HAPPY NEW YEAR.
DR. EDWARD O. AMPORFUL
CHIEF PHARMACIST
COCOA CLINIC


